The d- and f-Block Elements: Understanding the Transition and Inner-Transition Metals
In chemistry, the periodic table isn’t only a grid - it tells how substances act, interact, or shape things we see every day. Out of all sections, the d- and f-block ones catch attention thanks to unusual electron setups, vibrant chemicals, along with making many practical stuff.
These parts usually go by the name transition or inner-transition metals - yet they’re key in tech, living stuff, plus factories.
What Are d-Block Elements?
The d-block elements sit right in the middle of the periodic table - spanning groups 3 through 12.
They are called transition metals because they show a gradual transition of properties from metallic to non-metallic on the table.
General Electronic Configuration
(n−1)d¹–¹⁰ ns¹–²
This implies electrons begin occupying the d-orbitals.
Key Characteristics of d-Block Elements
Variable Oxidation States
They might shed varying amounts of electrons, creating charged particles such as Fe²⁺ or Fe³⁺, along with Cu⁺ and also Cu²⁺.
Coloured Compounds
Their d-electrons take in light, so compounds show hues like the blue from Cu²⁺ or the green found in Ni²⁺.
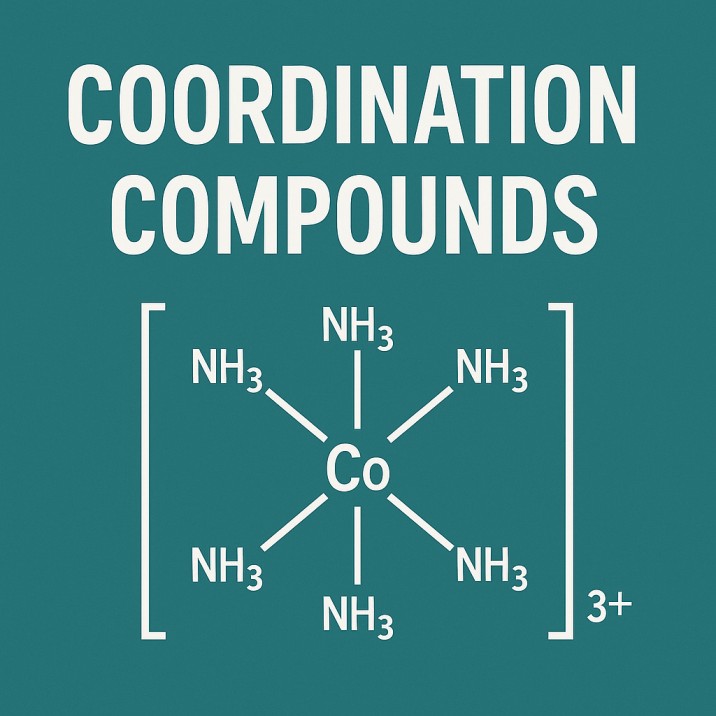
Formation of Complexes
They quickly link up with stuff like NH₃, H₂O, or Cl⁻ - making intricate structures along the way.
Magnetic Properties
Ferromagnetic behavior in elements such as iron, cobalt, or nickel comes from electrons that aren't paired up.
Catalytic Behaviour
Some metals help speed up reactions. Like how iron works in making ammonia, while nickel helps add hydrogen to oils.
What Are f-Block Elements?
The f-block items sit apart underneath the periodic chart; these consist of:
Lanthanides (58–71)
Actinides (90–103)
They're called inner-transition metals since the final electrons go into f-orbitals instead.
General Electronic Configuration
(n−2)f¹–¹⁴ (n−1)d⁰–¹ ns²
Characteristics of f-Block Elements
1. Lanthanides
They’re gentle metals that you’ll find recognized by:
Lanthanide shrinkage - atoms get smaller step by step
High reactivity
Colourful ions
Found in tough long-lasting magnets, laser tech, or old-school TV displays
2. Actinides
These parts contain dense minerals such as uranium, thorium - also plutonium. What stands out about them:
Mostly radioactive
Display various levels of electron loss
Fuel rods power reactors. Meanwhile, beams treat cancer. Also, labs run tests using particles
Applications of d- and f-Block Elements
Those things aren't just ideas - they're all over the place, right where you stand.
d-Block Applications
Iron: Steel production, construction
Copper: Electrical wiring
Titanium: Aerospace, prosthetics
Platinum, Palladium, Nickel: Catalysts in industries
f-Block Applications
Lanthanides power LED lights - also found in speaker magnets, while showing up in hybrid car batteries too
Actinides: Uranium in nuclear fuel, Thorium in reactors
Why These Elements Are Important
The d- and f-block components play a key role in:
Modern technology
Scans showing what’s inside your body plus ways doctors fix issues
Energy production
Electronics and communication
Environmental catalysts
Their knack for creating intricate ion structures, vibrant colors, how they react, along with their magnetism - these traits turn transition metals into chemistry’s most adaptable players.
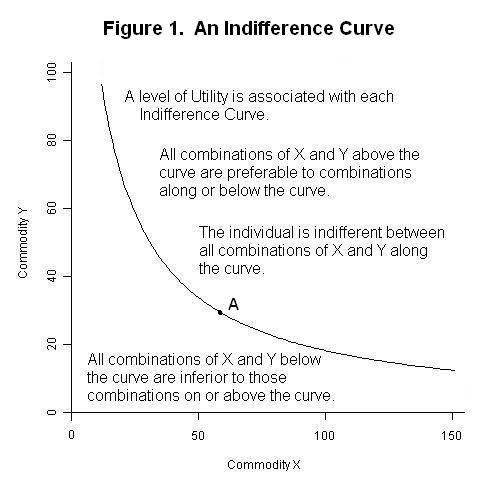
:max_bytes(150000):strip_icc():format(webp)/marginalutility-cff85ddfd620484f8afbf9d3f6cf4b74.jpg)
:max_bytes(150000):strip_icc():format(webp)/TermDefinitions_Utility-e42a7528caa347f9b1af149065ab2b9d.jpg)