Electrochemistry: Understanding the Science Behind Electricity and Chemical Reactions
The subject of electrochemistry sounds complicated until one grasps the core idea; thereafter, everything falls into place. In plain words, electrochemistry is the study of chemical reactions that produce electricity and electricity-driven chemical reactions. This is the branch of chemistry that describes the working of batteries, the purification of metals, and even why corrosion occurs.
What is electrochemistry?
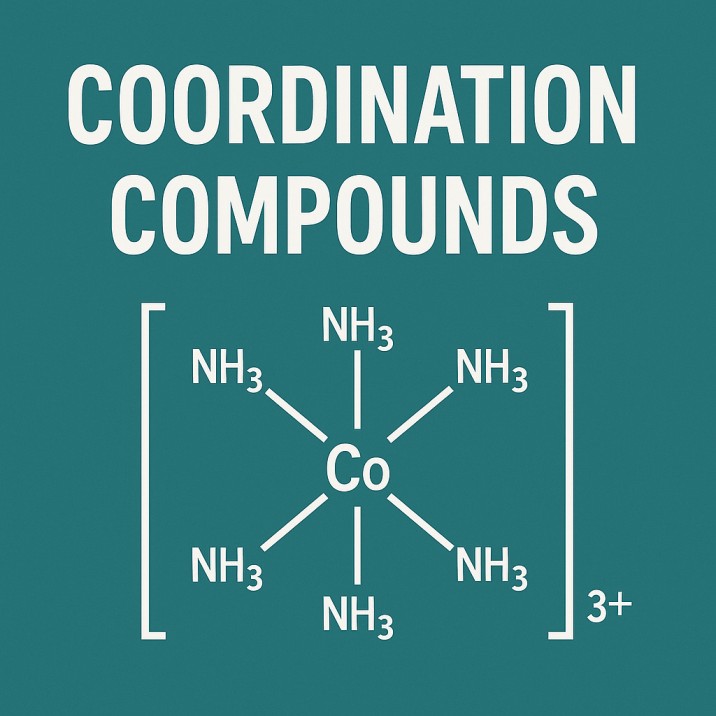
Electrochemistry is the study of the chemical reactions associated with the movement of electrons. Whenever electrons move from one substance to another, we can either generate electrical energy or use electrical energy to cause a reaction to occur.
Electrochemistry is based on two major ideas:
1. Oxidation – losing electrons
2. Reduction - gain of electrons
These always occur together, called redox reactions.
Electrochemical Cells: Where the Magic Happens
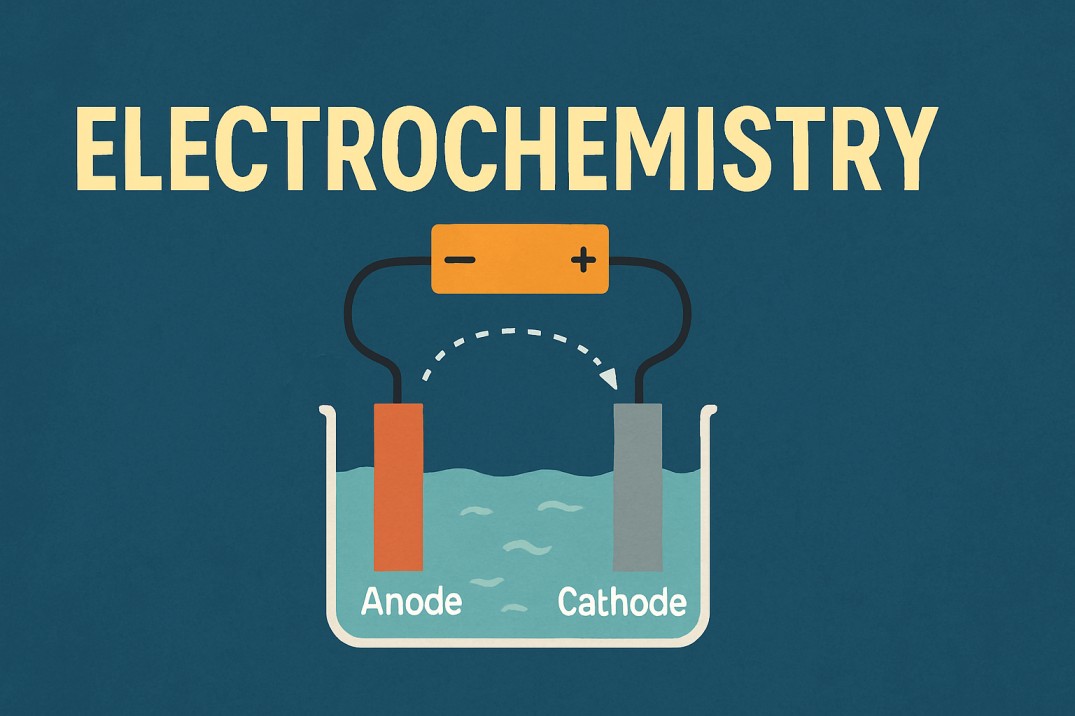
Electrochemical reactions occur in special settings called electrochemical cells, and there are two major types:
1. Galvanic (Voltaic) Cells
These cells produce electricity through a chemical reaction.
A good example is the battery in your phone or remote. Two different metals inside react with an electrolyte. When electrons move from one electrode to another, electrical energy is produced.
Key features:
Chemical energy → Electrical energy
Spontaneous reaction
Anode is negative, cathode is positive
2. Electrolytic Cells
These cells use electricity to force a reaction to occur.
Electrolytic cells are used in various processes such as electroplating, purification of metals, and splitting of water.
Key features:
Electrical energy → Chemical energy
Non-spontaneous reaction
Anode is positive, cathode is negative
These are the files that will be used when creating snapshots in Alfresco.
Electrodes and Electrolytes: The Key Players
Every electrochemical cell contains the following components:
Electrode
A solid conductor where the reaction occurs:
Anode – Where oxidation occurs
Cathode - where reduction takes place
(Remember it this way: AnOx, RedCat — Oxidation at Anode, Reduction at Cathode.)
Electrolyte
Solution or molten substance containing ions that assist the passage of electricity. Without ions, electrons would not move smoothly.
---
Applications of Electrochemistry in Daily Life
Electrochemistry is all around, not just in a textbook.
1. Batteries
Your phone, your laptop, your inverter, your car-everything uses electrochemical reactions to store and release energy.
2. Electroplating
Electrolysis is used in the jewelry coating, chrome plating of cars, and gold plating on electronics to deposit a thin layer of metal.
3. Corrosion Prevention
Basically, rusting is an unwanted electrochemical reaction. Techniques like galvanization protect metals.
4. Metal Extraction and Purification
Electrochemical principles play the main role in processes like the extraction of aluminium, refining copper, and a few more in industries.
5. Fuel Cells
These cells generate electricity cleanly by combining hydrogen and oxygen, a promising green technology.
-
Electrode Potential and EMF
Every metal has a tendency either to lose or to gain electrons. And this tendency is measured as its electrode potential.
The voltage produced due to the difference in potentials of two different metals when connected together is called electromotive force.
This EMF determines the strength and the speed at which a cell can generate electricity.
--- Why Electrochemistry Matters Electrochemistry bridges chemistry and physics, but most importantly, it connects science with real-life technology. From powering devices to developing clean energy solutions, electrochemistry is shaping the future. Moreover, it helps students to understand concepts such as redox reactions, flow of electrons, and chemical energy, which are very frequently asked about in competitive examinations.
:max_bytes(150000):strip_icc():format(webp)/marginalutility-cff85ddfd620484f8afbf9d3f6cf4b74.jpg)
:max_bytes(150000):strip_icc():format(webp)/TermDefinitions_Utility-e42a7528caa347f9b1af149065ab2b9d.jpg)